祝贺齐国栋、褚月英在Angew. Chem. Int. Ed杂志上发表文章
日期:2020/7/3 12:57:35 浏览量:次
Abstract
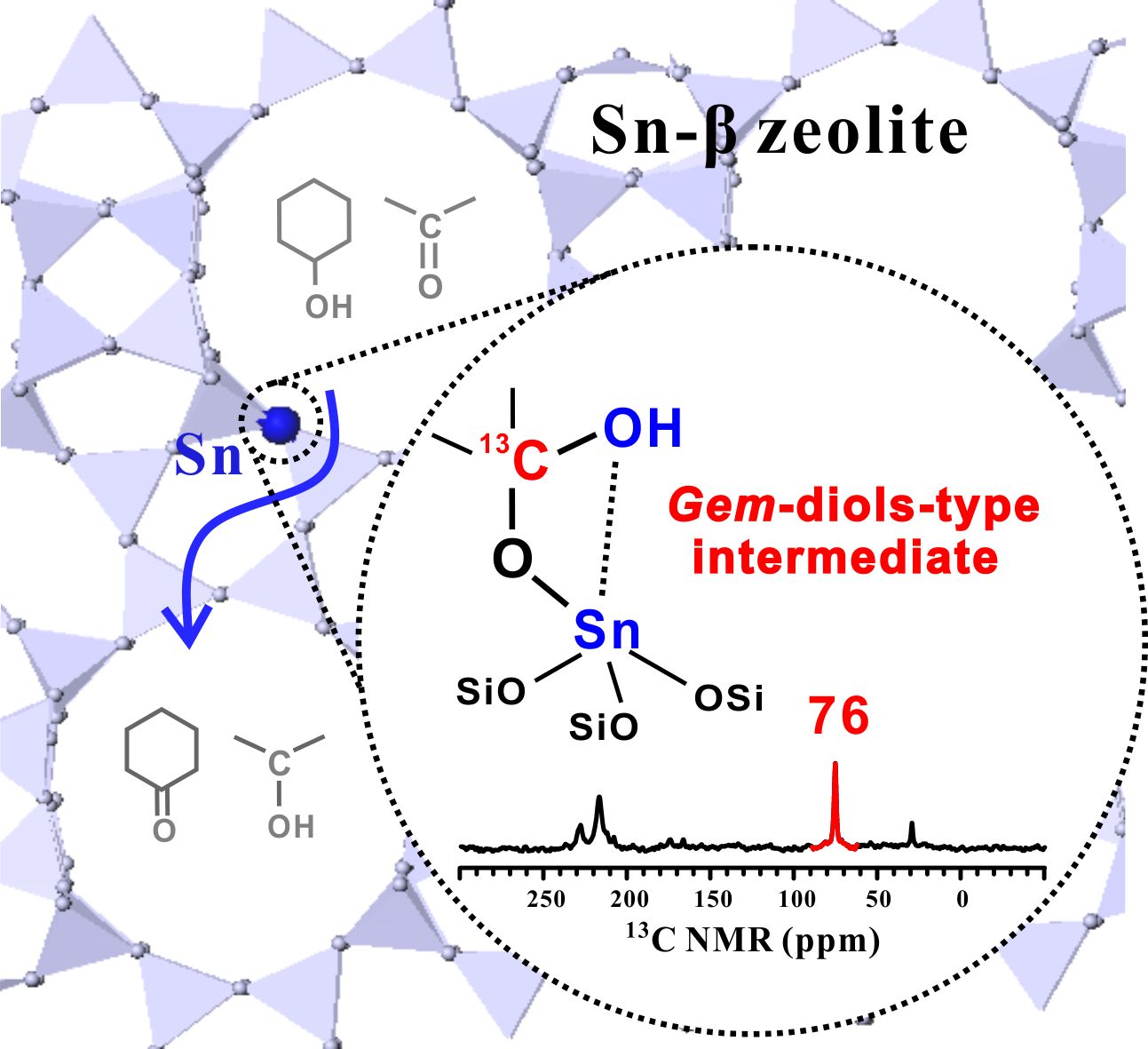
Lewis acid zeolites have found increasing application in the field of biomass conversion, in which the selective transformation of carbonyl‐containing molecules is of particular importance due to their relevance in organic synthesis. Mechanistic insight into the activation of carbonyl groups on Lewis acid sites is challenging and critical for the understanding of the catalytic process, which requires the identification of reaction intermediates. Here we report the observation of a stable surface gem‐diol‐type species in the activation of acetone on Sn‐β zeolite. 13C, 119Sn, and 13C–119Sn double‐resonance NMR spectroscopic studies demonstrate that only the open Sn site ((SiO)3Sn‐OH) on Sn‐β is responsible for the formation of the surface species. 13C MAS NMR experiments together with density functional theory calculations suggest that the gem‐diol‐type species exhibits high reactivity and can serve as an active intermediate in the Meerwein—Ponndorf–Verley–Oppenauer (MPVO) reaction of acetone with cyclohexanol. The gem‐diol‐type species offers an energy‐preferable pathway for the direct carbon‐to‐carbon hydrogen transfer between ketone and alcohol. The results provide new insights into the transformation of carbonyl‐containing molecules catalyzed by Lewis acid zeolites.
文章链接https://onlinelibrary.wiley.com/doi/10.1002/anie.202005589