Theoretical Chemical Calculations in Catalytic Reactions
Integrating ab initio thermodynamics, molecular dynamics, and microkinetic modeling approaches, the focus is on elucidating the specific structures and evolution processes of catalyst active centers under operating conditions. This multifaceted approach enables the examination of the mechanisms governing the adsorption, diffusion, and transformation of reactants on catalysts. The goal is to establish a clear relationship between the microstructure of catalysts and the underlying catalytic reaction mechanisms at the atomic-molecular level. By doing so, a comprehensive theoretical framework can be developed to study and understand complex catalytic processes more thoroughly.
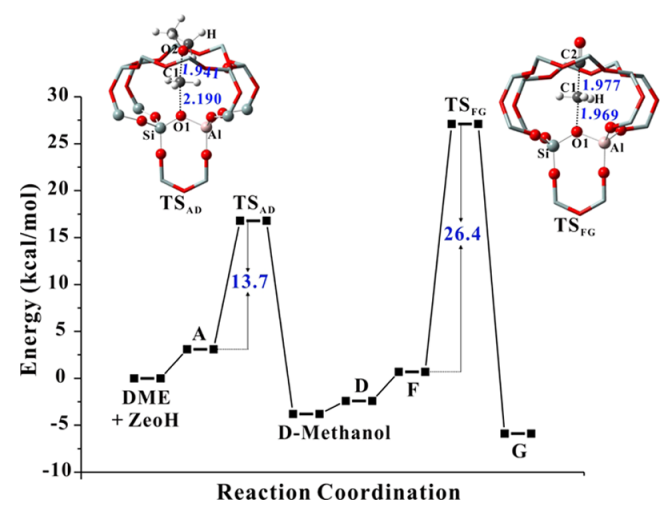
1. Y. Chu; X. Yi; C. Li; X. Sun; A. Zheng, Brønsted/Lewis acid sites synergistically promote the initial C–C bond formation in the MTO reaction. Chem. Sci. 2018, 9, 6470-6479.
2. Y. Chu; A.-Y. Lo; C. Wang; F. Deng, Origin of High Selectivity of Dimethyl Ether Carbonylation in the 8-Membered Ring Channel of Mordenite Zeolite. J. Phys. Chem. C 2019, 123, 15503-15512.
3. Y. Chu; G. Li; L. Huang; X. Yi; H. Xia; A. Zheng; F. Deng, External or internal surface of H-ZSM-5 zeolite, which is more effective for the Beckmann rearrangement reaction? Catal. Sci. Technol. 2017, 7, 2512-2523.



