Establishing a Link Between the Dual Cycles in Methanol-to-Olefins Conversion on H-ZSM-5: Aromatization of Cycloalkenes
Date:2020/5/12 14:41:15 Views:Times
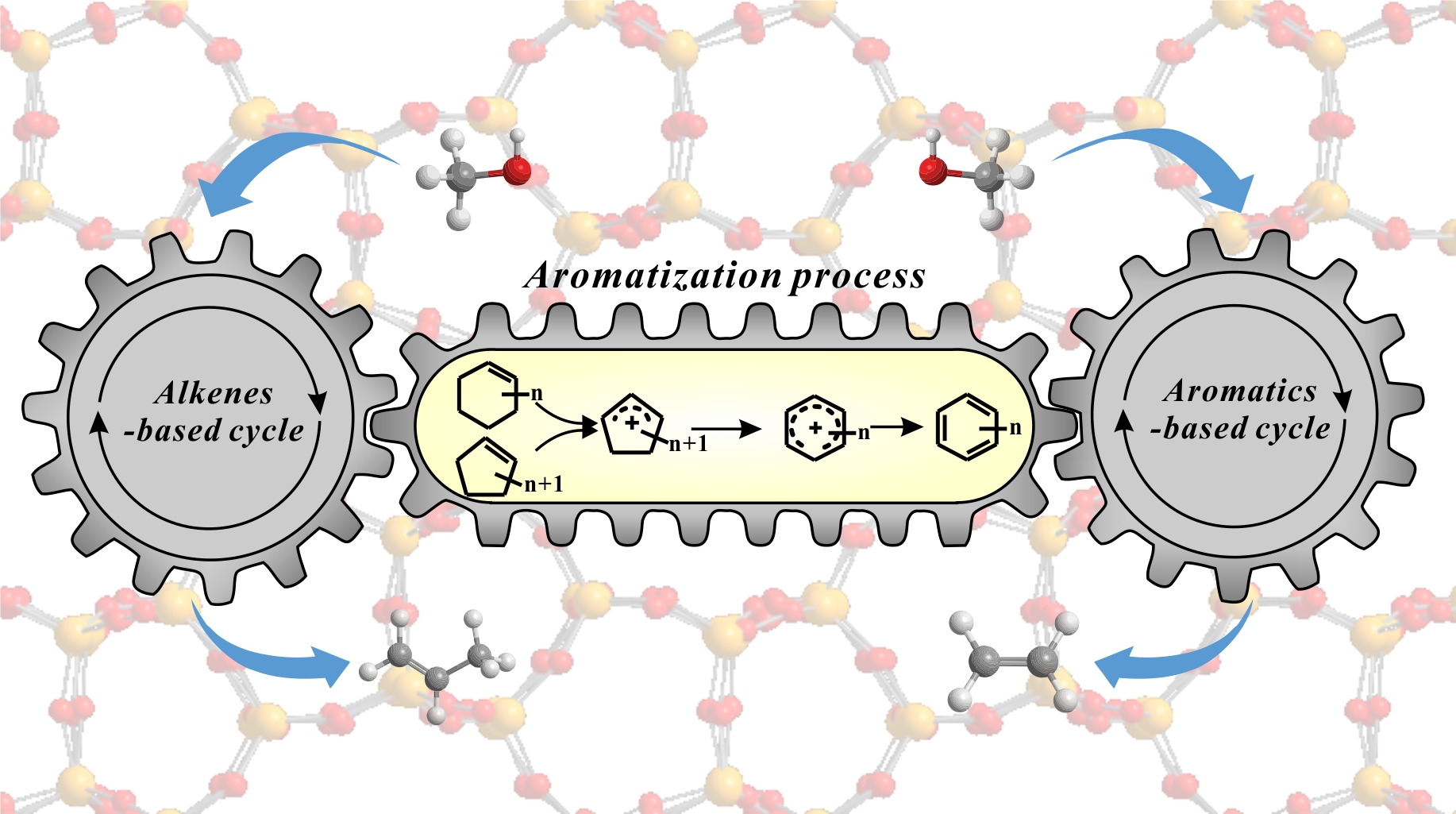
The aromatization of alkenes in the methanol-to-olefins (MTO) reaction on H-ZSM-5 zeolites was investigated by solid-state NMR and GC-MS spectroscopy. The formed cycloalkenes including cyclopentene and cyclohexene in the MTO reaction show high reactivity toward formation of aromatics. Cyclohexene tends to form cyclopentene through ring contraction, and the subsequent ring expansion leads to the production of aromatics. The ring-contraction/expansion process of cyclohexene is consistent with the 13C isotopic scrambling in aromatics. Solid-state NMR experiments evidenced the formation of cyclopentenyl cations and benzenium ions intermediates in the aromatization of C6 cycloalkenes. A plausible route to link the alkene-based and aromatic-based cycles in the MTO reaction on ZSM-5 zeolite was proposed, which is corroborated by DFT calculations.
Link:https://pubs.acs.org/doi/10.1021/acscatal.0c00838



