Direct Detection of Supramolecular Reaction Centers in the Methanol-to-Olefins Conversion over Zeolite H-ZSM-5
Date:2016/9/27 16:45:35 Views:Times
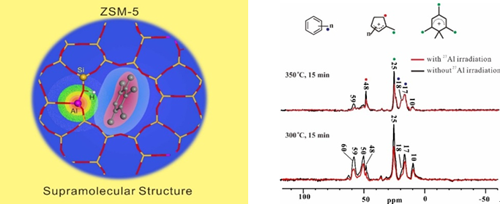
Hydrocarbon-pool chemistry is important in methanol to olefins (MTO) conversion on acidic zeolite catalysts. The hydrocarbon-pool (HP) species, such as methylbenzenes and cyclic carbocations, confined in zeolite channels during the reaction are essential in determining the reaction pathway. Herein, we experimentally demonstrate the formation of supramolecular reaction centers composed of organic hydrocarbon species and the inorganic zeolite framework in H-ZSM-5 zeolite by advanced 13C-27Al double-resonance solid-state NMR spectroscopy. Methylbenzenes and cyclic carbocations located near Brønsted acid/base sites form the supramolecular reaction centers in the zeolite channel. The internuclear spatial interaction/proximity between the 13C nuclei (associated with HP species) and the 27Al nuclei (associated with Brønsted acid/base sites) determines the reactivity of the HP species. The closer the HP species are to the zeolite framework Al, the higher their reactivity in the MTO reaction.
Link:https://onlinelibrary.wiley.com/doi/full/10.1002/anie.201510920



