Acidic Properties and Structure–Activity Correlations of Solid Acid Catalysts Revealed
Date:2016/4/25 16:46:02 Views:Times
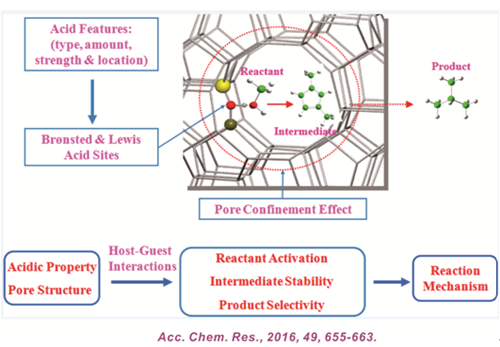
Solid acid materials with tunable structural and acidic properties are promising heterogeneous catalysts for manipulating and/or emulating the activity and selectivity of industrially important catalytic reactions. On the other hand, the performances of acid-catalyzed reactions are mostly dictated by the acidic features, namely, type (Brønsted vs Lewis acidity), amount, strength, and local environment of acid sites. The latter is relevant to their location (intra- vs extracrystalline), and possible confinement and Brønsted–Lewis acid synergy effects that may strongly affect the host–guest interactions, reaction mechanism, and shape selectivity of the catalytic system. This account aims to highlight some important applications of state-of-the-art solid-state NMR (SSNMR) techniques for exploring the structural and acidic properties of solid acid catalysts as well as their catalytic performances and relevant reaction pathway invoked. In addition, density functional theory (DFT) calculations may be exploited in conjunction with experimental SSNMR studies to verify the structure–activity correlations of the catalytic system at a microscopic scale.
We describe in this Account the developments and applications of advanced ex situ and/or in situ SSNMR techniques, such as two-dimensional (2D) double-quantum magic-angle spinning (DQ MAS) homonuclear correlation spectroscopy for structural investigation of solid acids as well as study of their acidic properties. Moreover, the energies and electronic structures of the catalysts and detailed catalytic reaction processes, including the identification of reaction species, elucidation of reaction mechanism, and verification of structure–activity correlations, made available by DFT theoretical calculations were also discussed. Relevant discussions will focus primarily on results obtained from our laboratories in the past decade, including (i) quantitative and qualitative acidity characterization utilizing assorted probe molecules, (ii) probing the spatial proximity and synergy effect of acid sites, and (iii) influence of acid features and pore confinement effect on catalytic activity, transition-state stability, reaction pathway, and product selectivity of solid acid catalysts such as zeolites, metal oxides, and heteropolyacids. It is conclusive that a synergy of acidity (local effect) and pore confinement (environmental effect) tend to strongly dictate the formations of intermediates and transition states, hence, the reaction pathways and catalytic performance of solid acid catalysts. We hope that these information can provide additional insights toward our understanding in heterogeneous catalysis, especially the roles of structural and acidic properties on catalytic performances and reaction mechanism of acid-catalyzed systems, which should be beneficial for rational design of solid acid catalysts.
Link:https://pubs.acs.org/doi/abs/10.1021/acs.accounts.6b00007



