Revealing the Bro̷nsted Acidic Nature of Penta-Coordinated Aluminum Species in Dealuminated Zeolite Y with Solid-State NMR Spectroscopy
Date:2024/9/30 20:26:28 Views:Times
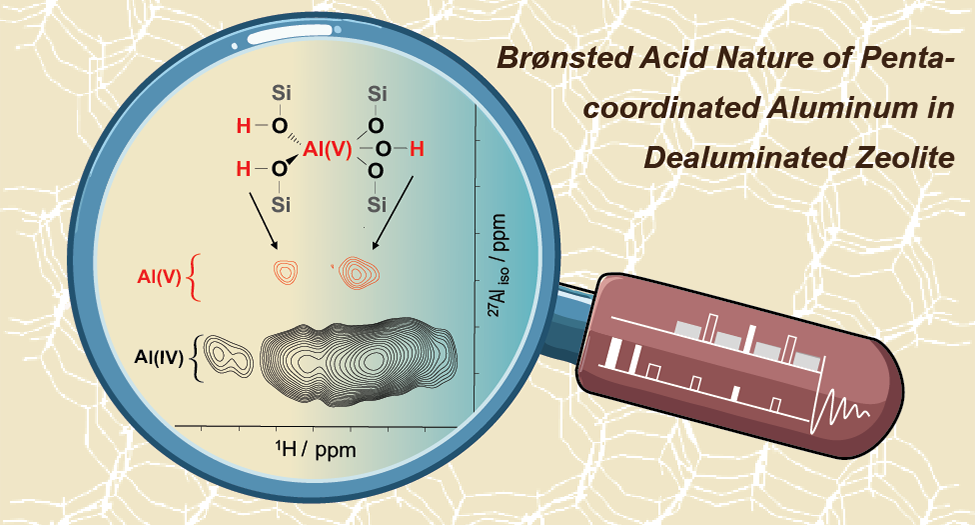
The inevitable dealumination process of zeolite Y is closely related to ultrastabilization, enhanced Bro̷nsted acidity, and deactivation throughout its life cycle, producing complex aluminum and acidic hydroxyl species. Most investigations on dehydrated zeolites have focused on the Bro̷nsted acidity of tetra-coordinated Al (Al(IV)) and Lewis acidity associated with tricoordinated Al (Al(III)) sites, which has left the penta-coordinated Al (Al(V)) in dealuminated zeolites scarcely discussed. This is largely due to the oversimplified view of detectable Al(V) as an exclusively extra-framework species with Lewis acidity. Here we report the formation of Bro̷nsted acidic penta-coordinated Al species (Al(V)-BAS) in the dealumination process. Two-dimensional (2D) through-bond and multiquantum 1H-{27Al} correlation solid-state NMR experiments demonstrate the presence of a bridging Si–OH–Al(V) structure in dealuminated Y zeolites. Different from the conventional belief that water attack leads to the breaking of zeolite framework Al–O bonds in the initial stage of zeolite dealumination, the observed Al(V) as a dealumination intermediate is directly correlated with a BAS pair because of the direct dissociation of water on the framework tetrahedral aluminum (Al(IV)), thus bypassing the cleavage of Al–O bonds. 1H double-quantum solid-state NMR experiments and theoretical calculations provide further evidence for this mechanism, whereas pyridine adsorption experiments confirm stronger acidity of Al(V)-BASs than the traditional bridging hydroxyl groups associated with Al(IV). We were also able to detect the Al(V)-BAS site from dealuminated SSZ-13 zeolite with CHA topology, suggesting that its creation is not specific to the framework structure of zeolites.
Link:https://pubs.acs.org/doi/10.1021/jacs.4c08408



