Unveiling the Brønsted acid mechanism for Meerwein–Ponndorf–Verley reduction in methanol conversion over ZSM-5
Date:2024/10/22 19:18:45 Views:Times
Abstract
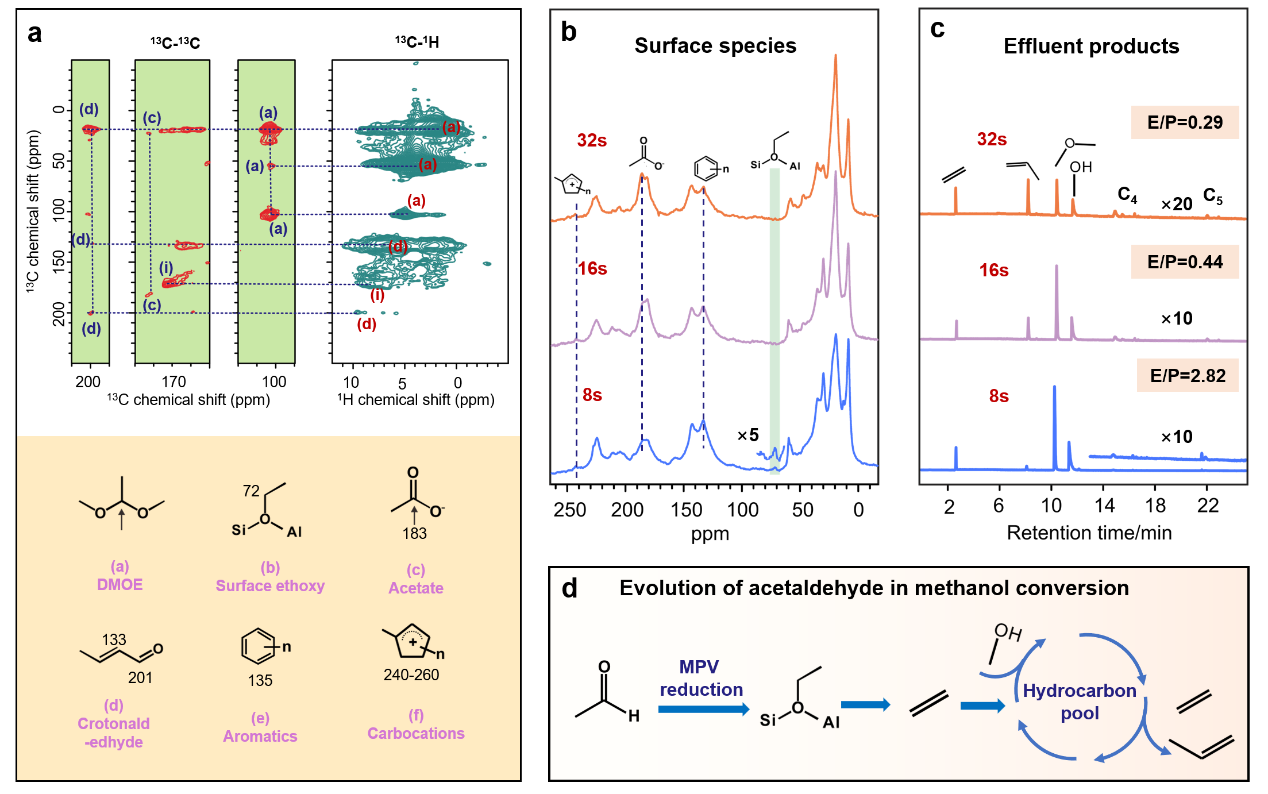
The conversion of methanol over zeolites offers a sustainable alternative for fuels and chemicals production. However, a complete understanding of the competing reaction pathways, particularly those leading to C-C bond formation, remains elusive. This work presents a novel mechanism for selective methanol conversion in ZSM-5 zeolites, involving a Brønsted acid site (BAS)-mediated Meerwein–Ponndorf–Verley (MPV) reduction pathway. Employing a multidimensional solid-state NMR spectroscopy combined with isotopic labeling and theoretical calculations, we identify this pathway for acetaldehyde reduction with methanol, directly contributing to ethene formation. This mechanism, involving carbenium ion intermediates like 1-hydroxyethane or 1-methoxyethane ions, contrasts with the well-established Lewis acid-catalyzed MPV process. Based on reactant adsorption modes, we propose two distinct reaction routes for BAS-MPV reduction, bridging the gap between direct and hydrocarbon pool mechanisms in methanol conversion. We further demonstrate the applicability of this pathway to acetone, highlighting its broader role in the early stages of the reaction.